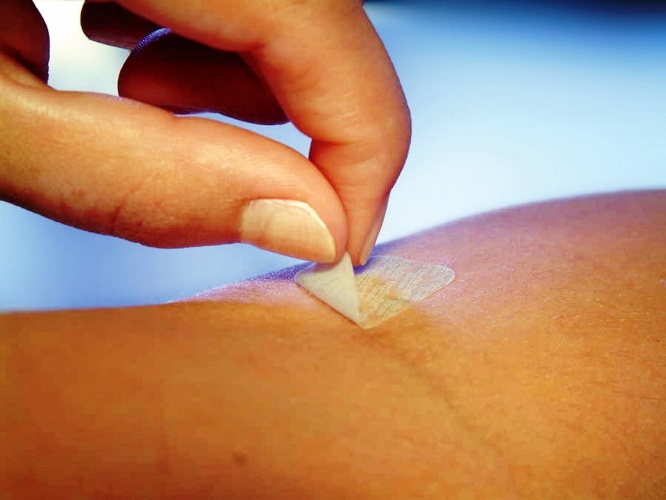
Wilmington, October 19, 2023 : DuPont (NYSE:DD) today announced the introduction of its new DuPont™ Liveo™ MG 7-9960 Soft Skin Adhesive. The higher-adhesion, low-cyclics silicone soft skin adhesive (SSA) is designed for advanced wound care dressings and adhering medical devices to the skin for long wear time and gentle removal.
DuPont™ Liveo™ has been a globally recognized leader in silicone technology for medical devices, setting the critical quality attributes of silicone skin adhesives. These technologies have become widely adopted by the market in advanced wound care and scar therapies, and now DuPont is complementing its historical range with this new SSA solution.
New DuPont™ Liveo™ MG 7-9960 Soft Skin Adhesive has the highest peel adhesion in the Liveo™ SSA family. It offers better conformability, atraumatic removal, high repositionability, improved wear performance and design flexibility. In addition, low-cyclics Liveo™ MG 7-9960 SSA is non-sensitizing, non-irritating and non-cytotoxic, making it ideally suited to protect the highly sensitive and fragile skin of populations including children, the elderly, and patients with skin conditions or open wounds.
The adhesive was developed as DuPont works to expand its portfolio of solutions to support the growing medical device market. It also allows manufacturers to fine-tune performance by varying coat weight to meet device designers’ applications requirements.
DuPont is sharing more information on Liveo™ MG 7-9960 Soft Skin Adhesive during an October 26 webinar hosted by Business Review Webinars. The complimentary 60-minute session will highlight how Liveo™ MG 7-9960 SSA enables advanced wound care and wearable device OEMs to extend dressing and patch offerings with improved wear performance and design flexibility. Webinar information can be found by visiting pmi-live.com and entering “9960” in the keyword search field.
“As difficult-to-heal wounds are increasingly prevailing and wound-healing rates need to improve, we are looking at expanding our portfolio of medical-grade adhesive solutions and partnering with medical device OEMs and converters to support them in developing their new dressing or wearables designs,” said DuPont™ Liveo™ Healthcare Solutions Global Strategic Marketing Leader Jennifer Gemo, one of the webinar presenters.
Because each device design and process “is specific to each customer, it is critical to not only supply high-quality, high-performance materials, but also to support customers in optimizing their development,” said DuPont™ Liveo™ Healthcare Solutions EMEA Technical Service and Development Professional and Medical Device Expert Audrey Wipret, who will co-present the webinar with Gemo.
In November, the DuPont™ Liveo™ Healthcare Solutions team will share developmental collaboration opportunities and product information at Stand L01 in Hall 08B at COMPAMED 2023 and Stand D33 in the Wearable Technologies Pavilion in Hall 12 at MEDICA 2023, both of which will take place Nov. 13 through 16 in Dusseldorf, Germany.
Corporate Comm India (CCI Newswire)